Mystery of “inactive” defensin solved:
Defense mechanism against bacteria and fungi deciphered
20.01.2011, Press releases
To defend microbial attacks, the human body naturally produces a group of antibiotics, called defensins. An interdisciplinary team of biochemists and medical scientists has now deciphered the mechanism of action of a defensin, hitherto looked upon as exhibiting only minor activity. Their results might be useful in future drug development for inflammatory and infectious diseases. Nature now presents their findings online ahead of the print publication.
Under standard laboratory conditions, the human beta-defensin 1 (hBD 1), a human antibiotic naturally produced in the body, had always shown only little activity against microbes. Nevertheless the human body produces it in remarkable quantities. The solution to the puzzle was the investigation process itself, as the research group led by Dr. Jan Wehkamp at the Dr. Margarete Fischer-Bosch Institute for Clinical Pharmacology of the Stuttgart-based Robert Bosch Hospital found out.
Before the research group took a new approach to this research, defensins were usually tested in the presence of oxygen, although little oxygen is present, for example, in the human intestine. Starting out from the discovery that a special antibiotic-activating protein of the human body is diminished in patients with inflammatory bowel diseases, Crohn’s Disease and Ulcerative Colitis, the working group investigated how defensins act under low-oxygen conditions. During their investigations the scientists found out that under these conditions hBD 1 unfolds a strong antibiotic activity against lactic acid bacteria and yeast.
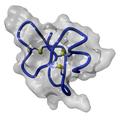
Furthermore the researchers discovered that another human protein, thioredoxin, is able to activate beta-defensin 1 even in the presence of oxygen. Moritz Marcinowski and Professor Johannes Buchner from the Department of Chemistry at the Technical University of Munich, used circular dichroism spectroscopy to elucidate the differences between the folded inactive and the unfolded active form of the protein.
Surprisingly, while almost all proteins are active only in their folded form, in the case of the small defensin the opposite is true. To activate the beta-defensin 1 the thioredoxin opens the three disulphide bridges that hold the molecule together. The molecule then opens up into the active state. Using this mechanism the body has the opportunity to selectively activate the defensin.
So far the cause of inflammatory bowel disease is unclear. Genetic as well as environmental factors seem to play a role, finally leading to a weakening of the antimicrobial barrier, which is mainly mediated by defensins. Accordingly the identified mechanism might contribute to the development of new therapies to treat affected patients.
The presented work is the result of a cooperation project with six participating centers, led by Emmy Noether junior research group leader Dr. Jan Wehkamp. In addition to five researchers from Stuttgart (Dr. Margarete Fischer-Bosch-Institute for Clinical Pharmacology and the Robert Bosch-Hospital (Bjoern Schroeder, Sabine Nuding, Julia Beisner, Eduard Stange and Jan Wehkamp) the Department of Dermatology at University of Tübingen (Martin Schaller), the Max-Planck-Institute for Developmental Biology in Tübingen (Sandra Groscurth), the Department of Dermatology at University of Kiel (Zhihong Wu), as well as the Department of Chemistry at the Technische Universitaet Muenchen (Moritz Marcinowski and Johannes Buchner) were involved. The work was funded by the Deutsche Forschungsgemeinschaft (Emmy Noether-Program for young researchers, Cluster of Excellence Center for Integrated Protein Science Munich) and by the Robert- Bosch-Foundation.
Original publication:
„Reduction of disulfide bonds unmasks potent antimicrobial activity of human β-defensin 1”, B.O. Schroeder, Z. Wu, S. Nuding, S. Groscurth, M. Marcinowski, J. Beisner, J. Buchner, M. Schaller, E.F. Stange, J. Wehkamp, Nature, DOI: 10.1038/nature09674
Contact:
Prof. Johannes Buchner
Department of Chemistry
Technische Universitaet Muenchen
Lichtenbergstr. 4, 85747 Garching, Germany
Tel: +49 89 289 13341 –Fax: +49 89 289 13345
E-mail
Priv. Doz. Dr. med. Jan Wehkamp
Dr. Margarete Fischer-Bosch-Institute for Clinical Pharmacology
Robert Bosch Krankenhaus, Internal Medicine I
Auerbachstr. 112; 70376 Stuttgart, Germany
Tel.: +49 711 8101-5700 – Fax: +49 711 85 92 95
E-Mail
Kontakt: presse@tum.de
More Information
| 110120_Buchner-HBD1_PW_DE.pdf |
Druckversion der Presseinformation (DE),
(Type: application/pdf,
Size: 109.2 kB)
Save attachment
|
|
| 110120_Buchner-hBD1_PW_EN.pdf |
Printable version of the press release (EN),
(Type: application/x-pdf,
Size: 106.0 kB)
Save attachment
|