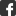Neues Angriffsziel für maßgeschneiderte Antibiotika entdeckt:
Sonderweg macht Bakterien angreifbar
Neues Ziel für Antibiotika: Der Eisen-Schwefel-Cluster (lila/gelb) im Inneren des IspH-Proteins von Bakterien.
02.07.2009, Pressemitteilungen
Immer mehr Bakterienstämme entwickeln Mehrfachresistenzen gegen die bisher lebensrettenden Antibiotika. Mediziner warnen, dass die Todesraten aufgrund von Infektionen schon in naher Zukunft dramatisch ansteigen könnten. Forscher der Technischen Universität München (TUM) haben nun einen Stoffwechselschritt aufgeklärt, der bei vielen aggressiven Mikroorganismen, wie dem Turberkulose- oder Malariaerreger vorkommt und deshalb ein lohnendes Ziel für eine neue Klasse von Antibiotika werden könnte. Die Ergebnisse ihrer Arbeit stellen die Wissenschaftler in der aktuellen Ausgabe der Zeitschrift Angewandte Chemie vor.
Antibiotika behindern die Produktion lebenswichtiger Stoffe in Mikroorganismen und halten die gefährlichen Eindringlinge so in Schach. Immer mehr Bakterienstämme entwickeln jedoch inzwischen Mehrfach-Resistenzen, sodass die bisher lebensrettenden Medikamente versagen. Daher suchen Wissenschaftler auf der ganzen Welt fieberhaft nach Reaktionsschritten, die für die zu bekämpfenden Mikroorganismen lebenswichtig sind, beim Menschen aber keine oder keine relevante Funktion haben. Ein Team um Professor Michael Groll, Dr. Jörg Eppinger und Dr. Tobias Gräwert, Biochemiker an der TU München, haben nun die strukturellen Grundlagen für einen solchen Reaktionsschritt detailliert beschrieben.
Die lebenswichtigen Naturstoffe aus der Terpen- und Steroidklasse stellen die Zellen fast aller Organismen aus den kleinen Isoprenbausteinen Dimethylallylpyrophosphat (DMAPP) und Isopentenylpyrophosphat (IPP) her. Säugetiere und eine große Zahl weiterer Organismen bauen diese über den so genannten Mevalonat-Weg auf. Fast alle krankmachenden Bakterien, so auch der Malariaerreger Plasmodium falciparum, haben dagegen eine andere Möglichkeit gefunden, diese wichtigen Substanzen herzustellen. Ihr Sonderweg könnte den Bakterien nun zum Verhängnis werden: Die TUM-Wissenschaftler haben die strukturellen Grundlagen des letzten Schritts der bakteriellen Isopren-Synthese aufgeklärt. Das entscheidende Enzym verfügt über eine äußerst ungewöhnliche Struktur, ähnlich der eines dreiblättrigen Kleeblatts. Es könnte sich als entscheidender Angriffspunkt für neue, maßgeschneiderter Antibiotika erweisen.
Die Arbeiten zur Aufklärung der bakteriellen Isoprenbaustein-Produktion wurden bereits vor zwölf Jahren am Lehrstuhl für Organische Chemie und Biochemie durch Professor Adelbert Bacher in Zusammenarbeit mit den Privatdozenten Wolfgang Eisenreich und Felix Rohdich begonnen. Im Laufe der Jahre entdeckte das Team die meisten Reaktionsschritte des neuen Stoffwechselwegs. Der letzte vom Enzym IspH katalysierte Schritt entzog sich jedoch hartnäckig der strukturellen Aufklärung. Die früheren Messungen legten nahe, dass das aktive Zentrum ein Eisen-Schwefel-Cluster mit drei Eisen und vier Schwefelatomen sein müsste. Andere Wissenschaftler zweifelten die Ergebnisse an, und jahrelang gelang es nicht, eine Kristallstruktur des Enzyms zu bestimmen, die den Beweis hätte liefern können.
Größtes Problem dabei war die Sauerstoffempfindlichkeit des Enzyms, das an der Luft schon in kürzester Zeit degeneriert und dabei Struktur und Funktion einbüßt. Erst kürzlich gelang es einer Arbeitsgruppe von der Justus-Liebig-Universität in Gießen, die Röntgenkristallstruktur der offenen Form des Enzyms zu entschlüsseln. Doch diese Struktur lieferte kaum Informationen zum Ablauf der vom Enzym katalysierten Umsetzung. Das Forscherteam um Professor Groll, Dr. Eppinger und Dr. Gräwert gelang es nun, auch die Röntgenkristallstruktur der geschlossenen Form zu lösen, die die genaue Faltung der Proteinkette und die chemische Umgebung des aktiven Zentrums zeigt.
Nun konnten sie durch Computersimulationen und Mutagenese-Experimente, bei denen Escherichia coli-Bakterien dazu gebracht wurden fehlerhafte IspH-Enzyme zu synthetisieren, den Mechanismus der Reaktion detailliert untersuchen. Röntgenkristallstruktur, kinetische Messungen und Mutagenesestudien bestätigten schließlich die schon vor Jahren vorgeschlagene, ungewöhnliche Anordnung von drei Eisen und vier Schwefelatomen im aktiven Zentrum des Enzyms.
„Nachdem nun Ort, chemischer Ablauf und beteiligte Partner der IspH-Reaktion bekannt sind,“ erläutert Groll, „besteht ein neuer Ansatzpunkt gezielt Substanzen zu entwickeln, die den letzten Schritt der bakteriellen Synthese von Isoprenbausteinen blockieren und Erreger gezielt abtöten könnten. Da Enzym und Reaktion in Säugetieren nicht vorkommen, sollten diese Verbindungen für Menschen keine oder nur geringe Nebenwirkungen besitzen.“
Die Arbeiten wurden unterstützt von der Hans-Fischer-Gesellschaft und dem Stifterverband für die Deutsche Wissenschaft (Projekt-Nr. 11047: Forschungsdozentur Molekulare Katalyse).
Originalpublikation:
Das IspH-Protein von Escherichia coli – Struktur und Mechanismus;
Tobias Gräwert, Felix Rohdich, Ingrid Span, Adelbert Bacher, Wolfgang Eisenreich, Jörg Eppinger, and Michael Groll
Angew. Chem., Vol. 121, Issue 18, 12165-12177, June, 2009, DOI 10.1002/ange.200900548
Link: http://www3.interscience.wiley.com/journal/122474116/abstract
Bildmaterial:
http://mediatum2.ub.tum.de/?cunfold=800265&dir=800265&id=800265
Kontakt:
Prof. Dr. Michael Groll
Technische Universität München
Department Chemie
Lichtenbergstr. 4, D-85748 Garching
Tel.: +49 89 289 13360
Fax: +49 89 289 13363
E-Mail - Internet
Kontakt: presse@tum.de
Mehr Information
| 090702_isph_pw |
Presseinformation,
(Type: application/pdf,
Größe: 174.1 kB)
Datei speichern
|
|
| 090702_IspH_PW_EN.pdf |
Press release,
(Type: application/pdf,
Größe: 44.6 kB)
Datei speichern
|