Wissenschaftler von eigenen Ergebnissen überrascht:
Entstehungsmodell für Amyotrophe Lateralsklerose in Frage gestellt
06.03.2012, Pressemitteilungen
Auch eine gut begründete Hypothese kann sich als wissenschaftliche Sackgasse erweisen – wie ein aktuelles Bespiel aus der medizinischen Forschung zeigt. Die Amyotrophe Lateralsklerose (ALS) ist eine Erkrankung des zentralen Nervensystems, die zu einer fortschreitenden Muskellähmung führt. Über eine mögliche Krankheitsursache waren sich die Wissenschaftler bisher weitgehend einig: Ein Transportproblem zentraler Zellbestandteile (Organellen) des Stoffwechsels lässt Axone von motorischen Neuronen verkümmern, die wiederum den Muskelapparat des Menschen steuern. Neue Untersuchungen eines Forscherteams rund um Prof. Thomas Misgeld von der Technischen Universität München (TUM) stellen jetzt dieses Erklärungsmodell in Frage.
Ein typisches Merkmal neurogenerativer Erkrankungen ist eine Schädigung von Axonen, bis zu einem Meter lange, dünne Fortsätze von Nervenzellen. Wie eine Art Kabel leiten sie elektrische Signale innerhalb eines einzelnen Neurons weiter – bis zu den Synapsen, die dann die Kommunikation zwischen den Nervenzellen übernehmen. Bisher gingen Wissenschaftler davon aus, dass der gestörte Transport von Zellorganellen entlang der Axone unmittelbar zu ihrem Absterben führt, da Organellen wie Mitochondrien für den Stoffwechsel und -austausch in der Zelle verantwortlich sind.
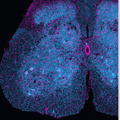
Die Wissenschaftler untersuchten Axone auf morphologische Veränderungen und Störungen im Zelltransport. Unter dem Mikroskop verfolgten sie die Bewegungen einzelner Mitochondrien, den Energielieferanten der Zelle, und von Vesikeln des Endosom-Systems, dem „Speditionsunternehmen“ der Zelle. Zu ihrer eigenen Überraschung stellten sie fest, dass die eingeschränkte Bewegung der Organellen und das Axon-Sterben voneinander unabhängige Prozesse darstellen können – und widerlegten so die bis dahin gültige Hypothese.
Zusammen mit seinem Kollegen Prof. Martin Kerschensteiner von der Ludwig-Maximilians-Universität führte Misgeld umfangreiche Tests an Tiermodellen mit verschiedenen genetischen Mutationen durch, die ALS beim Menschen auslösen. Die beiden Wissenschaftler stützten sich dabei auf ein neuartiges Bildgebungsverfahren, wie Misgeld erklärt: „Für unsere Arbeit entwickelten Martin Kerschensteiner und ich neue Methoden, um die Organellen im Axon mittels genetischer Verfahren zu markieren und unter speziellen ‚Zeitraffer’-Mikroskopen live zu beobachten. Wir haben damit verschiedene ALS-Varianten untersucht. Da wir davon ausgegangen waren, dass allen Krankheitsmodellen ein Transportproblem der Organellen zugrunde liegt, traf uns die Beobachtung, dass verschiedene Modelle sich in dieser Hinsicht unterscheiden, völlig unvorbereitet.“
Offensichtlich gibt es für die eingeschränkte Mobilität der Organellen und für das Absterben der Axone unterschiedliche Mechanismen. Zumindest im Fall der ALS scheint der Zelltransport daher nicht das geeignete Ziel für therapeutische Ansätze darzustellen. „Wir denken, dass unsere Erkenntnisse Folgen für künftige ALS-Studien, vielleicht sogar für Untersuchungen anderer neurogenerativer Erkrankungen wie Alzheimer oder Chorea Huntington haben werden. Das Beispiel der ALS-Hypothese zeigt, wie schwierig es ist, zuverlässige kausale Modelle für dieses Krankheitsbild zu erstellen – und dass wir unsere Annahmen sehr sorgfältig prüfen müssen.“
Originalpublikation:
Axonal transport deficits and degeneration can evolve
independently in mouse models of amyotrophic lateral sclerosis
Petar Marinković, Miriam S. Reuter, Monika S. Brill, Leanne
Godinho, Martin Kerschensteiner, and Thomas Misgeld, PNAS Early Edition, Feb.
27-March 2, 2012.
DOI:10.1073/pnas.1200658109
Multimedia-Download:
http://mediatum.ub.tum.de/?cfold=1098037&dir=1098037&id=1098037
Kontakt:Prof. Dr. Thomas Misgeld
Institute of Neuroscience
Technische Universität München
Biedersteinerstrasse 29
80802 München
Tel.: +49.089.4140-3512
E-Mail -
Web
Kontakt: presse@tum.de
Mehr Information
| 120305_ALS-Hypothese.pdf |
Druckversion der Presseinformation (DE),
(Type: application/pdf,
Größe: 95.2 kB)
Datei speichern
|