Munich researchers discover new disease mechanism
Early Damage in multiple sclerosis can be reversed
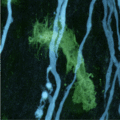
In multiple sclerosis, immune cells (green) attack nerve-cell projections or axons (blue). Light microscopy picture of the spinal cord of a mouse.
27.03.2011, News
Munich Reseachers have found a previously unknown mechanism which explains how nerve-cell projections (axons) are destroyed in multiple sclerosis (MS). In an animal model of MS, this process is reversible if recognized and treated early. Therefore it could serve as a target for future therapeutic intervention. The results are published in the online-version of Nature Medicine at 11/03/27.
Multiple sclerosis is a common and, in many cases seriously disabling, autoimmune disease that can lead to the disturbance or loss of sensory function, voluntary movement, vision and bladder control. Commonly, it is thought that the primary target of MS is the myelin sheath, an insulating membrane that enwraps axons, and increases the speed of signal transmission. However, damage to nerve fibers is also a central process, as whether autoimmune pathology ultimately leads to permanent disability depends largely on how many nerve fibers are damaged over the course of time.
The team led by Kerschensteiner and Misgeld set out to define precisely how the damage to the nerve axons occurs. As Misgeld explains, “We used an animal model in which a subset of axons is genetically marked with a fluorescent protein, allowing us to observe them directly by fluorescence microscopy.” After inoculation with myelin, these mice begin to show MS-like symptoms. But the researchers found that many axons showing early signs of damage were still surrounded by an intact myelin sheath, suggesting that loss of myelin is not a prerequisite for axonal damage.
Chemical radicals attack axons
Instead a previously unrecognized mechanism, termed focal axonal degeneration (FAD), is responsible for the primary damage. FAD can damage axons that are still wrapped in their protective myelin sheath. This process could also help explain some of the spontaneous remissions of symptoms that are characteristic of MS. “In its early stages, axonal damage is spontaneously reversible,” says Kerschensteiner. “This finding gives us a better understanding of the disease, but it may also point to a new route to therapy, as processes that are in principle reversible should be more susceptible to treatment.”
However, one must remember that it takes years to transform novel findings in basic research into effective therapies. First the process that leads to disease symptoms must be elucidated in molecular detail. In the case of MS it has already been suggested that reactive oxygen and nitrogen radicals play a significant role in facilitating the destruction of axons. These aggressive chemicals are produced by immune cells, and they disrupt and may ultimately destroy the mitochondria. Mitochondria are the cell’s powerhouses, because they synthesize ATP, the universal energy source needed for the build-up and maintenance of cell structure and function.
Similar findings in patients
“In our animal model, at least, we can neutralize these radicals and this allows acutely damaged axons to recover,” says Kerschensteiner. The results of further studies on human tissues, carried out in collaboration with specialists based at the Universities of Göttingen and Geneva, are encouraging. The characteristic signs of the newly discovered process of degeneration can also be identified in brain tissue from patients with MS, suggesting that the basic principle of treatment used in the mouse model might also be effective in humans.
Even if this turns out to be the case, it would not mean that a new therapy would soon be at hand. The chemical agents used in the mouse experiments are not specific enough and not tolerated well enough to be of clinical use. “Before appropriate therapeutic strategies can be developed, we need to clarify exactly how the damage arises at the molecular level,” says Kerschensteiner. “We also want to investigate whether similar mechanisms play a role in later chronic stages of multiple sclerosis.”
Funding:
The work received generous support from the Deutsche Forschungsgemeinschaft (DFG), in the context of Sonderforschungsbereich 571 (Autoimmune disease: From symptoms via mechanism to therapy) and the Emmy Noether Program. The Hertie Foundation, the largest private supporter of brain research in Germany, and the Alexander von Humboldt Foundation also contributed significantly to financing the project. The study was performed within the framework of the Center for Integrated Protein Science Munich (CIPSM) -- a Cluster of Excellence -- and the Multiple Sclerosis Competence Network set up by the Federal Ministry for Research and Technology. Prof. Thomas Misgeld is Fellow at the Institute for Advanced Study of the Technical University of Munich, a think tank created with support of the German Excellence Initiative.
Publication:
http://www.nature.com/nm/journal/vaop/ncurrent/index.html
Contact:
Prof. Thomas Misgeld
Chair of Biomolecular Sensors
Institute of Neuroscience
at Technische Universitaet Muenchen
Phone.: 089 / 4140 3512
E-mail: thomas.misgeld@lrz.tum.de
Prof. Martin Kerschensteiner
Instititute of Clinical Neuroimmunology
Klinikum der Universitaet Muenchen
Phone: 089 / 2180 78282
E-mail: Martin.Kerschensteiner@med.uni-muenchen.de
Kontakt: presse@tum.de



